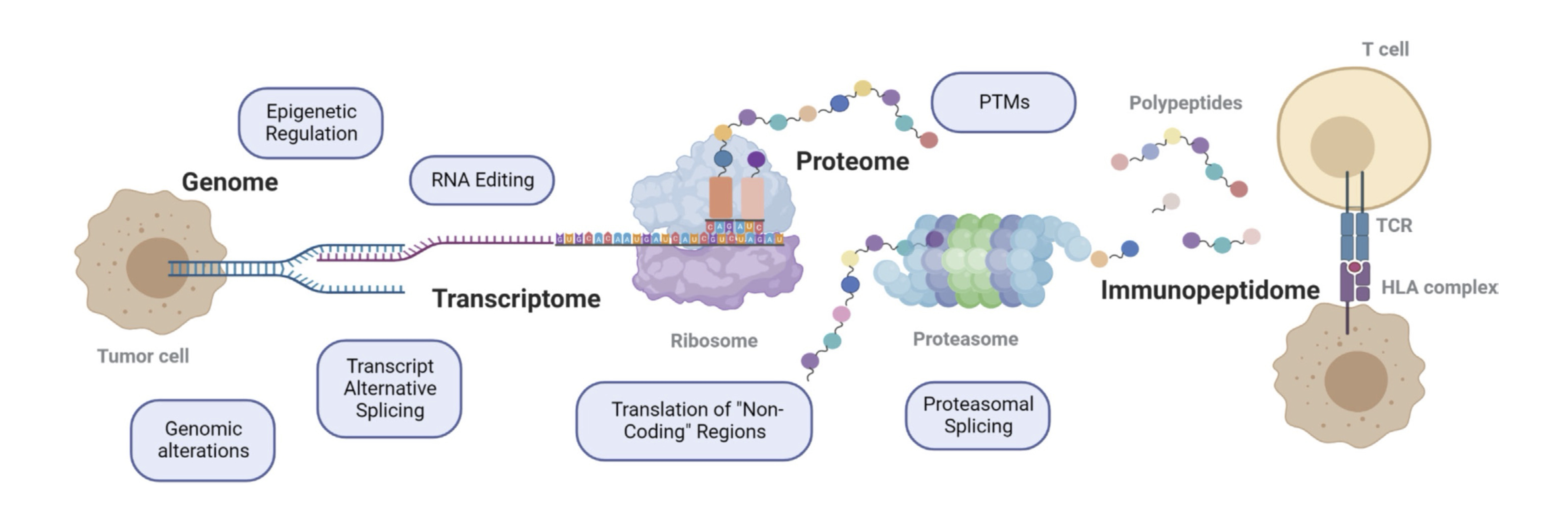
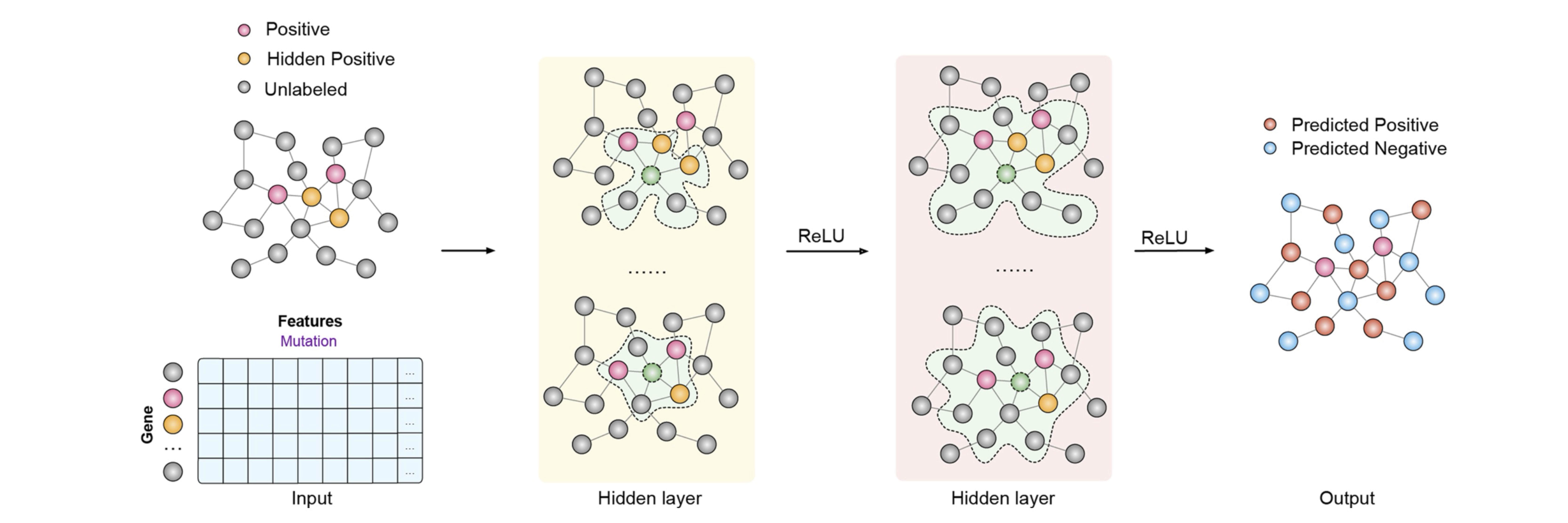
Welcome to Dr. Bing Zhang’s Lab at the Baylor College of Medicine. We develop and use integrative bioinformatics approaches to extract biological meanings from experimental data and generate hypotheses for experimental validation. Please explore our website to learn more about our people and our research.
Lab News
[2025-12] Thank you to our dedicated lab members and collaborators for an inspiring year of discovery. Wishing everyone a joyful holiday season and continued success in the coming year. 
[2025-10] QCB student Abigail Beman joined the group for a research rotation. Welcome, Abby!
[2025-10] CCB student Hannah Engebretson joined the group for a research rotation. Welcome, Hannah!
[2025-09] Our collaborative project with Drs. Chonghui Cheng and Jeroen Pollet, titled “mRNA-based immunotherapy for targeting triple-negative breast cancer“, has received a V Foundation award through the Stuart Scott Memorial Cancer Research Fund.
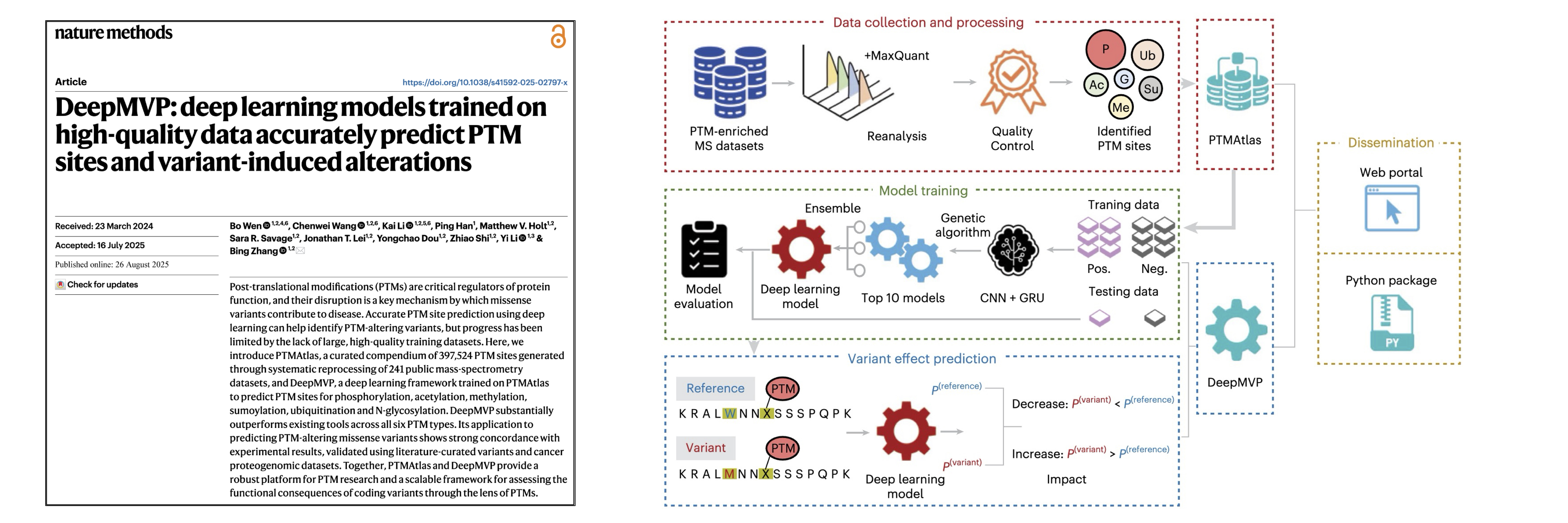
[2025-08] Our paper DeepMVP: deep learning models trained on high-quality data accurately predict PTM sites and variant-induced alterations has been published in Nature Methods. Congratulations to Bo, Chenwei, Kai, and all co-authors! This study introduces PTMAtlas, a curated compendium of 397,524 PTM sites generated through systematic reprocessing of 241 public mass-spectrometry datasets, and DeepMVP, a deep learning framework trained on PTMAtlas to predict PTM sites for phosphorylation, acetylation, methylation, sumoylation, ubiquitination and N-glycosylation. Together, they provide a robust platform for PTM research and a scalable framework for assessing the functional consequences of coding variants through the lens of PTMs. PTMAtlas, DeepMVP and a Python package for seamless integration of DeepMVP into genomics pipelines are available at http://deepmvp.ptmax.org. Among the various reports covering this study, two that appear to be AI-generated are particularly effective in making the work easily accessible and engaging to a broad audience: a youtube video and a Chinese article.
[2022-08] Our collaborative paper with Dr. Seth Lerner titled “Proteogenomic characterization unveils biomarkers associated with chemoresistance in muscle-invasive bladder cancer” has been published in Cell Reports Medicine. Congratulations to Matt, Yongchao, and all co-authors!
[2025-08] We have received funding from Pfizer for a project titled “Enhancing Proteogenomics Workflows for Drug Target Identification.”
[2025-07] Our collaborative project with Dr. Chonghui Cheng’s lab, titled “Targeting Cryptic Splicing-Derived Neoantigens in hnRNPM-Dysregulated Triple-Negative Breast Cancer,” has been selected for a DoD Breakthrough Award.
[2025-07] Drs. Zhang and Dou have been awarded an MPI U01 grant from the NCI’s Informatics Technology for Cancer Research (ITCR) program for their project titled “Advancing Protein Isoform Analysis through an Integrated Computational Framework.”
[2022-06] The CPTAC study “Proteogenomic analysis of the CALGB 40601 (Alliance) HER2+ breast cancer neoadjuvant trial reveals resistance biomarkers” has been published in Cell Reports Medicine. Congratulations to Eric and all co-authors!
[2022-05] QCB student Fanwei Ruan joined the group as a graduate student. Welcome, Fanwei!
[2022-05] Our collaborative paper with Dr. Julio Saez-Rodriguez’s group titled “Comprehensive evaluation of phosphoproteomic-based kinase activity inference” has been published in Nature Communications. Congratulations to Eric, Sophia, and all co-authors!
[2025-04] Our lab participated in the 19th Annual Breast Center Retreat, gave oral and poster presentations, and won the group karaoke competition! 
[2025-03] Lihong Xu from the Immunology & Microbiology Program joined the group for a research rotation. Welcome, Lihong!
[2025-03] Faye’s paper Deciphering the dark cancer phosphoproteome using machine-learned co-regulation of phosphosites has been published in Nature Communications. Congratulations! This study introduces CoPheeMap, a network built from phosphoproteomic data across 11 cancer types that maps how over 26,000 phosphorylation sites are co-regulated. Using this network, we further developed CoPheeKSA, a machine learning model that predicts which kinases modify which phosphosites. The model uncovered over 24,000 kinase–substrate relationships, including many involving poorly understood sites and kinases. Validated with experimental data, these tools help reveal cancer-related signaling changes and highlight potential new drug targets. The code for CoPheeMap and CoPheeKSA is available on GitHub at: https://github.com/bzhanglab/CoPheeMap.
[2025-02] Congratulations to Michael for passing the CCB PhD Qualifying Exam!
[2025-01] CCB student Shiyi Wu joined the group for a research rotation. Welcome, Shiyi!
[2025-01] QCB student Fanwei Ruan joined the group for a research rotation. Welcome, Fanwei!
[2024-12] Holiday party at Topgolf. 
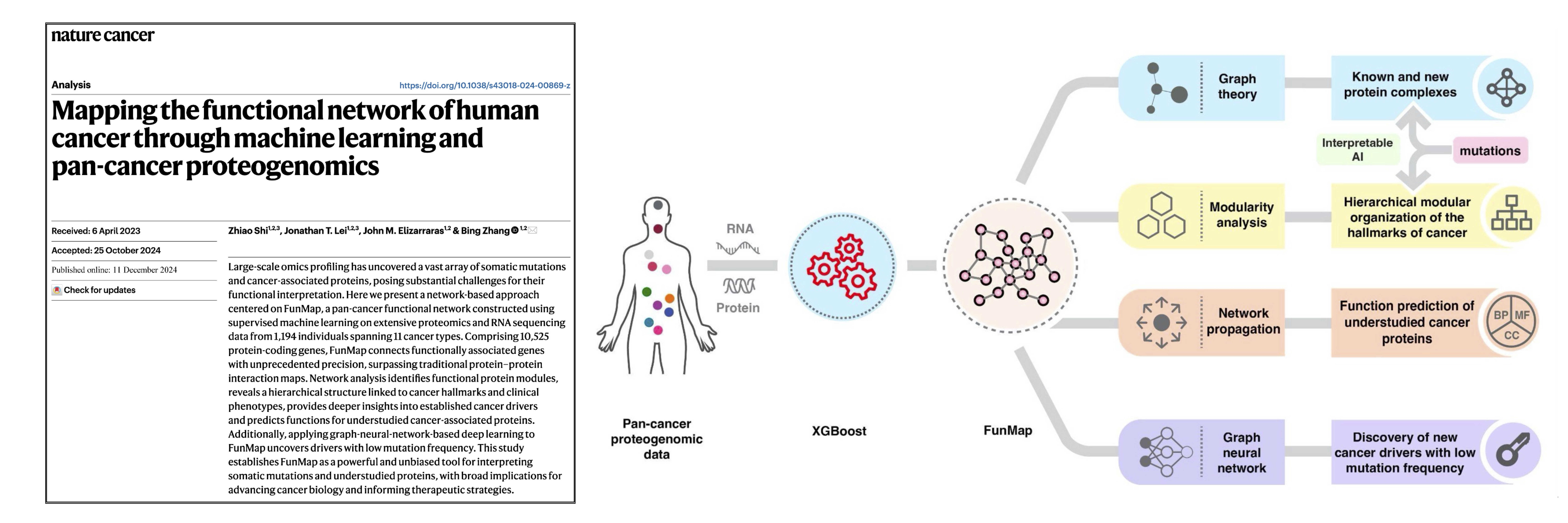
[2024-12] The paper Mapping the functional network of human cancer through machine learning and pan-cancer proteogenomics has been published in Nature Cancer. Congratulations to co-first authors Zhiao and Jonathan! By applying supervised machine learning to extensive proteomics and RNA sequencing data from 1,194 individuals spanning 11 cancer types, this study constructs a functional network, FunMap, comprising 10,525 protein-coding genes. FunMap achieves unprecedented precision in linking functionally associated genes, surpassing traditional protein-protein interaction maps. Analysis of FunMap identifies functional protein modules, reveals a hierarchical structure linked to cancer hallmarks and clinical phenotypes, provides deeper insights into established cancer drivers and predicts functions for understudied cancer-associated proteins. Additionally, applying graph-neural-network-based deep learning to FunMap uncovers drivers with low mutation frequency. The FunMap Python package is fully open source and available for download from the Python Package Index (https://pypi.org/project/funmap). The source code is hosted on GitHub (https://github.com/bzhanglab/funmap). In addition, a web application, accessible at https://funmap.linkedomics.org/, offers visualization tools to explore gene neighborhoods, dense modules, and the hierarchical organization of this pan-cancer FunMap.
[2024-12] James successfully defended his PhD thesis, congratulations, Dr. Moon! He will be joining Sage Bionetworks next month. Best wishes for a bright future!
[2024-11] Dr. Moran Chen joined the lab as a postdoctoral research associate. Welcome, Moran!
[2024-11] Xinpei’s paper Tumor-associated antigen prediction using a single-sample gene expression state inference algorithm has been published in Cell Reports Methods. This study introduces a Bayesian-based algorithm for inferring gene expression states in individual samples. By integrating this algorithm into a computational workflow for tumor-associated antigen (TAA) identification, the study uncovers TAAs shared across tumors within and between cancer types, including a repository of experimentally validated peptides to support further immunotherapy development for liver cancer.
[2024-10] Congratulations to Jonathan on starting his new role as an Instructor at the Breast Center!
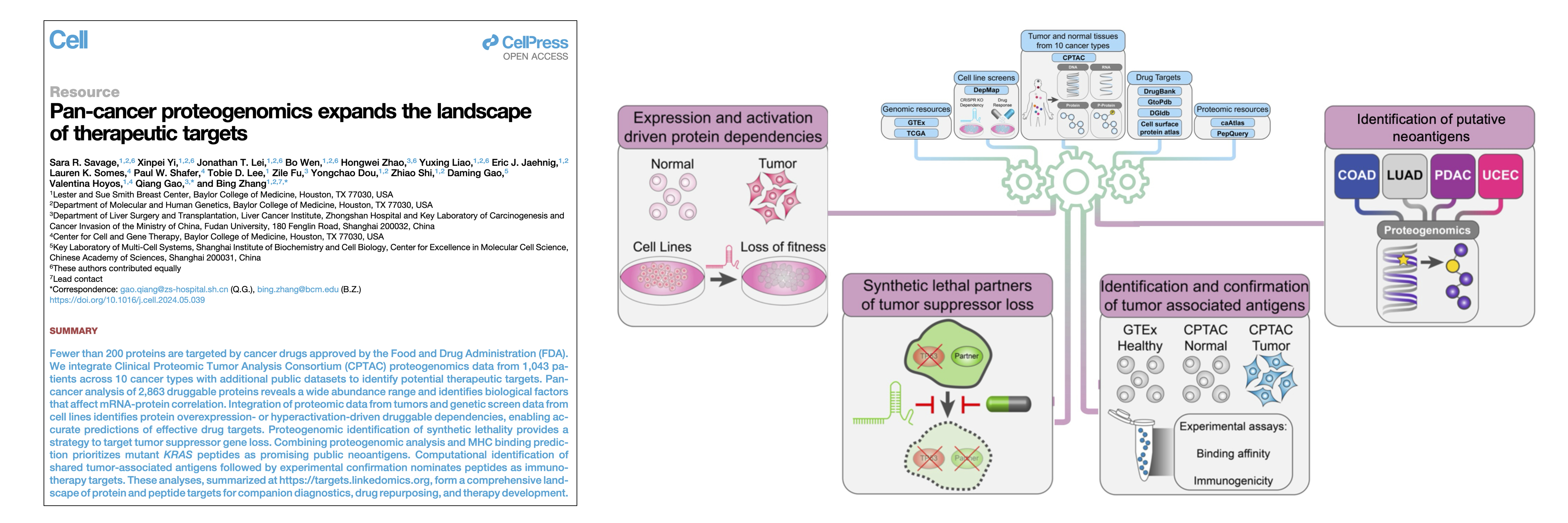
[2024-08] The paper Pan-cancer proteogenomics expands the landscape of therapeutic targets has been published in Cell! Congratulations to co-first authors Sara, Xinpei, Jonathan, Bo, Hongwei, and Yuxing, as well as the Gao and Valentina labs for their outstanding experimental validation of the predicted targets. This study integratesClinical Proteomic Tumor Analysis Consortium (CPTAC) proteogenomics data from 1,043 patients across 10 cancer types with additional public datasets to identify potential oncology targets. These include overexpressed and hyperactivated protein dependencies, protein dependencies associated with the loss of tumor suppressor genes, and putative neoantigens and tumor-associated antigens. These analyses, summarized at https://targets.linkedomics.org, form a comprehensive landscape of protein and peptide targets for companion diagnostics, drug repurposing, and therapy development.
[2024-08] QCB student Siyu Wang joined the group for a research rotation. Welcome, Siyu!
[2024-08] Congratulations to Yongchao on the promotion to Assistant Professor at the Breast Center!
[2024-07] John’s paper WebGestalt 2024: faster gene set analysis and new support for metabolomics and multi-omics has been published in Nucleic Acids Research. The 2024 WebGestalt update marks a major advancement, introducing support for metabolomics, streamlined multi-omics analysis capabilities, and substantial performance improvements enabled by a Rust-based implementation. Discover these updates and more at https://www.webgestalt.org.
[2024-05] CCB student Michael Lee joined our group and Dr. Cheng’s lab as a graduate student, co-mentored by Dr. Zhang and Dr. Cheng. Welcome, Michael!
[2024-04] Dr. Zhang received the Michael E. Debakey Excellence in Research Award. Congratulations!
[2024-04] Dr. Paul Shafer joined the lab as a postdoctoral research associate. Welcome, Paul!
[2024-03] Dr. Yanling Sun joined the lab as a postdoctoral research associate. Welcome, Yanling!
[2024-03] Seunghyuk, John, Lindsey, Chenwei and Bing attended the CPTAC symposium and US HUPO in Portland, OR. Congratulations to Chenwei on receiving the Meritorious Poster Award for his work on “DeepVEP: predict effects of variants on post-translational modifications with deep learning”. 
[2024-02] Drs. Zhang and Hoyos have been awarded a CPRIT grant in Computational and Systems Biology for their project titled “Charting TCR–Tumor Antigen Interactions to Foster Novel Immunotherapeutic Approaches for Triple-Negative Breast Cancer.”
[2024-02] Breast center Spring Festival celebration. 
[2024-01] Sara’s paper Frozen tissue coring and layered histological analysis improves cell type-specific proteogenomic characterization of pancreatic adenocarcinoma has been published in Clinical Proteomics. This study demonstrates the feasibility of multi-omics data generation from tissue cores, the necessity of interval H&E stains in serial histology sections, and the utility of coring to improve analysis over bulk tissue data.
[2023-12] Xinpei’s paper Deep Learning Prediction Boosts Phosphoproteomics-Based Discoveries Through Improved Phosphopeptide Identification has been published in Molecular & Cellular Proteomics. Shotgun phosphoproteomics enables high-throughput analysis of phosphopeptides in biological samples, but low phosphopeptide identification rates in data analysis limit its potential. This paper presents DeepRescore2, a computational workflow that leverages deep learning-based predictions of retention time and fragment ion intensity to enhance phosphopeptide identification and phosphosite localization. Benchmarking against existing workflows on a synthetic phosphopeptide dataset and application to real-world biological datasets demonstrate increased sensitivity, reduced missing values, and improved insights from phosphoproteomics-based biological analyses. DeepRescore2 is available at https://github.com/bzhanglab/DeepRescore2.
[2023-12] Holiday bowling party.